The last time we saw a molecular orbital view of molecules was when we looked at aldehydes and ketones. We will try to look at molecular orbitals whenever that look will help us to understand more about why organic molecules react in the way they do.

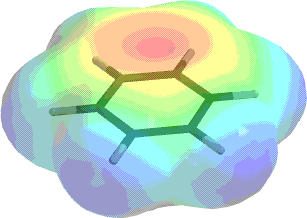
Benzene is a planar molecule with a very high electron density in the core (above and below the plane of the ring) and some relatively positively charged hydrogens around the perimeter. The diagram above shows the total electron density (out to a density value assumed for space-filling models). The red areas show a relatively high attraction for a point positive charge, while the blue areas show a small repulsion for the same charge. This indicates that the center of the ring is very electron-rich, while the outside is electron-poor.
We'll stick to the pi orbitals of benzene. There are three important bonding orbitals that are composed mostly of p-atomic orbitals, which all add up to make one donut of electron density above and one below the plane of the ring.
|
The lowest-lying pi molecular orbital is very symmetrical around the ring. There is a single node, as expected from its origin in p-atomic orbitals. This is the picture we usually get from the textbooks. |
|
|
These are the two orbitals which, having the same energy, could be called the HOMO's for benzene. Note that each has two nodes (one in the plane of the ring, and one perpendicular to that plane. These orbitals are still fairly symmetrical, having a mirror plane of symmetry about the nodes. |
|
|
This diagram shows both HOMO's together, illustrating the point that the sum of the HOMO's is actually a broad bonding coverage around the aromatic ring in a fashion quite similar to the lower lying pi orbital shown at the top. This view is also rotated to be looking straight on at the ring. |
|
The dominant reaction of the benzene nucleus is with electrophiles. We will examine these reactions in another page, still under development. Stay tuned....