Professor Richard Blatchly--Research
My general focus is on applying the tools of organic chemistry to the understanding of biological systems. Bioorganic chemistry is a powerful tool which has applications in drug development, design of catalysts for industrial processes, and fundamental study of living systems, to name a few.
Bioorganic chemists are often "general practice" chemists. One must know quite a bit about biological systems to get the inspiration for the projects, about organic synthesis to make new molecules for testing, about spectroscopy for testing the systems, and about theory for predicting what molecules to make, and how they behave on an atomic scale. Other inspirations come from architecture, woodworking, car repair and playing with childrens' toys.
One of the enduring chemical problems that is vital to the understanding of biological systems is the manner in which single, complicated molecules adopt a specific shape in solution, and also aggregate with other molecules to form a specific assembly. Proteins that mediate most of the everyday business of life are comprised of chains of small subunits. These chains fold into a single form, despite millions of possible shapes, so as to perform their function.
In addition to directly applying as many analytical and theoretical tools as possible to this problem, it often makes sense to mimic certain functions of biological systems with very different types of molecules. These mimics may be very poor, or relatively good. However, the specific advantages and deficits that we find compared to the authentic system help us gain insight into the workings of natural systems. In addition to gaining fundamental knowledge about natural systems, one might expect to find materials that perform non-biological functions, such as sensing the presence of toxins or making better electronic devices.
The process of producing these bio-mimics involves many phases. The work can be roughly broken up into three major portions: design and engineering, synthesis, and analysis. In brief, we make an educated guess about what will work a certain way; we make the molecules, using standard synthetic techniques; and we decide whether it did indeed work as we had intended. This cycle feeds data into the next design phase, so that with good fortune and diligence, we achieve our goal.
Currently, I am working on mimicking protein structure using unnatural substances. This mimicry is a bold and ambitious project, but has very substantial payoffs even if we never actually produce an artificial enzyme or receptor. It starts with molecules that fold in a predictable fashion: |
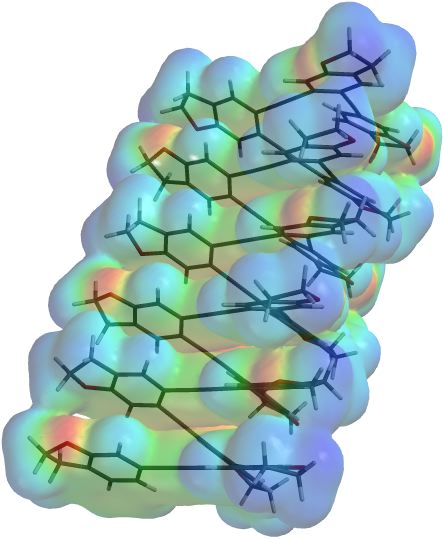
Ortho-phenylene ethynylene in its helix form. Molecules like this may form the structural elements of artificial biomolecules. We are still trying to prove that these molecules adopt a helical form, as we predict from theoretical studies. |
Foldamers
The study of foldamers has both practical and fundamental applications; the field holds promise for new medicines, materials, and greater insight into molecular folding. Foldamers can be described as flexible molecules that prefer to adopt one specific shape in solution. Gellman declared in 1998 that any large molecule with a strong tendency to adopt a specific compact conformation is a foldamer. Moore stated in 2001 that foldamers are any oligomer that folds into a conformationally ordered state in solution. These compact structures are stabilized by a collection of noncovalent interactions between nonadjacent monomer units. He continues that:
- Foldamers customarily have regular repeating structures (elements) within the backbone.
- Foldamers are often characterized by oligomeric, not polymeric, size.
- This means their respective molecular weights are typically in the range of 500 to 5,000 daltons.
The folding reaction is dynamic, meaning the molecule can unfold to adopt a set of random conformations and refold. Hence, molecules without conformational flexibility are not regarded as foldamers; this reaction also infers that association of multiple foldamer strands involves entropy loss similar to the folding reaction.
When folded, these molecules possess a unique set of atomic coordinates, or at the very least, a few sets of different coordinates.
Because these molecules adopt a compact or folded conformation in solution, the solvent may have an important influence on the folded state and the molecule's atomic coordinates. Noncovalent interactions between non-adjacent monomer units influence conformation and these are emphasized because although their relative strength is weak, the number of possible interactions is large and dependent on the molecule's length. In summation, native atomic coordinates are ultimately defined by noncovalent interactions.
This work is being done in collaboration with the Greg Tew group in the UMass Department of Polymer Science and Engineering. We have been synthesizing and studying phenylene ethynylene oligomers as biomimics for the last several years.
Design
The specific project on which Dr. Tew and I have been collaborating involves the synthesis of molecules which mimic proteins containing bundled helical substructures. The inspiration for this work comes from biopolymers like myoglobin, a protein commonly found in red meat, and synthetic peptide helix bundles developed to expand our understanding of why proteins fold. Naturally occuring foldamers have the following characteristics, which we would like to mimic: 1) A polymeric structure—chainlike, made up of similar repeating units; 2) Flexible shapes which tend to adopt specific shapes under the correct conditions; 3) The exterior of the specific shape is patterned to allow it to interact with other molecules of complementary pattern. This complementary nature is based on several characteristics: electric charge (positive attracts negative), strength of interaction with water (groups that interact weakly with water tend to group together), size and flexibility.
Making Foldamers
Synthesis of ortho-phenylene ethynylene molecules works in two stages. The oligomer (a short string of repeated structures) is assembled by a catalytic assembly reaction between two monomer molecules, appropriately protected. In principle, this allows us to plan for convergent synthesis of larger structures, by assembling a short stretch of structure (say, 3 repeats) and connecting it to another short stretch, making large changes in molecular size in one step. Once a library of small sequences is produced, a systematic study of the effects of sequence on structure and properties could be attempted.
Producing the monomers for assembly into short sequences is not trivial. Most require the application of traditional organic techniques over several sequential steps. We have performed several steps of monomer synthesis at Keene State. The availability of real synthetic space in our new building will make this job easier!
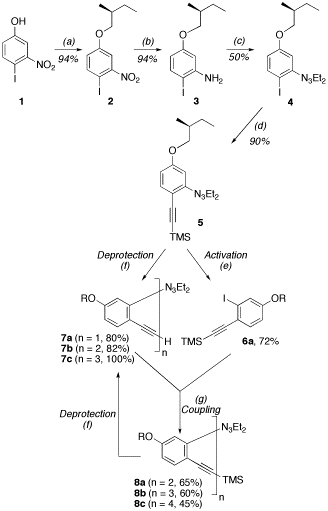
The synthesis for a hexamer is shown to the right.
The synthesis involves the production of pivotal monomer 5 , followed by a cycle of acetylene deprotection, triazene activation, and Sonogashira coupling to produce dimer 8a, trimer 8b, and tetramer 8c. Convergent coupling of appropriate trimer molecules (6b and 7c) produces hexamer 8d. This synthesis was published in Organic Letters in 2003
|
|
Theoretical Prediction
When synthetic techniques are developed, one of the most crucial early questions that must be answered in this system is how to control the tendency to fold into specific shapes (we are especially interested in helical, or corkscrew shapes) under the appropriate conditions by altering the chemical characteristics present in each molecule. A sample reaction is shown below in its simplest form; the tendency to form helices can be defined as the relative number of molecules in the helix form, compared to the number found in the extended forms. In 2003, I began to develop a theoretical framework for predicting this tendency of the molecules to form helices. A paper describing this work has published, in which we analyze the effects of changing the substituents attached to the outside of the main chain. There are a number of remaining questions, however, due to the complexity of the system proposed.
Folding Reaction

Once the tendency to form helices is relatively well-understood, the ability of these helices to pack into bundles can be studied. Initially, simple systems that pack into bundles of small numbers of helices will be studied to develop the preparation and analysis.
Methods for Analysis
Difficult problems like proving the existence of helical forms of a molecule will not typically yield to a single technique. A wide variety of techniques must be employed. These include NMR spectroscopy, infrared spectroscopy, fluorescence spectroscopy and circular dichroism studies.
Another collaboration which is gathering momentum is with Dr. Patricia O'Hara of the Amherst College Chemistry Department. She specializes in the fluorescence spectroscopy of biomolecules, and is helping us analyze the inherent fluorescence of these molecules. By using less common techniques like fluorescence anisotropy and fluorescence lifetime measurement, we are coming to a much greater understanding of the complex spectra that mixtures in solution can produce.
Synergy
Why do all this work, when one could have plenty to do with just one piece? The fact is that science work is not done in isolation. Our synthesis work tells us what molecules are reasonably available. The theoretical work tells us what molecules will be most likely to be successful in behaving as we hope. The analytical work tells us if our theoretical predictions are accurate, and may give us ideas about what new molecules to synthesize. Knowledge of any one portion of the project helps make work in the other aspects much more effective. This integration of knowledge from different areas of chemistry also helps us to stay true to the liberal arts mission at KSC.
Recent Publications
R. A. Blatchly and G. N. Tew, J. Org. Chemistry ,
68(23): 8780-5 (2003). “Theoretical Study of Helix Formation in Substituted Phenylene Ethynylene Oligomers”
Ticora V. Jones, Richard A. Blatchly and Gregory N. Tew, Org. Lett., 5(18): p. 3297-9 (2003). “Synthesis of Alkoxy-Substituted Ortho-Phenylene Ethynylene Oligomers”
Comments? Email me!